Whether wireless foot switches, mobile monitors or portable analysers: Where batteries are involved, the Battery Regulation (EU) 2023/1542 applies. August 2025 was an exciting month with new obligations for all market participants affected by the Battery Regulation. But watch out: With the Omnibus Package IV, obligations have been postponed. What do medical device manufacturers installing batteries in their devices have to consider as of now? We inform you about scope, obligations and deadlines.
What is the battery regulation for?
Batteries have become an integral part of our everyday clinical lives – from wireless foot switches to mobile monitors and smart data collectors. But what actually happens to these powerhouses when their life comes to an end? This is precisely where the EU Battery Regulation comes in, with the aim of making the entire life cycle of batteries environmentally friendly, socially responsible and safe. The regulation thus joins a series of EU environmental laws and at the same time expands the scope of your product compliance tasks.
- Regulation (EU) 2023/1542 was adopted by the European Parliament and the Council on 12 July 2023.
- Since 18 February 2024 is has already been applicable for the most part. From 18 August 2025, it will completely replace the previous Battery Directive 2006/66/EC.
- As a regulation, it applies directly in all EU member states.
- Many articles are supplemented by delegated acts.
- Application deadlines for due diligence obligations under Article 48 were postponed by Omnibus (EU) 2025/1561.
Who is affected by the Battery Regulation – explanation of roles
The Regulation places obligations on all economic actors along the supply chain – from battery manufacturers to distributors. These roles are relevant for medical technology:
- Producer
- importer
- distributors
- Manufacturer of devices with batteries
- Authorised representative
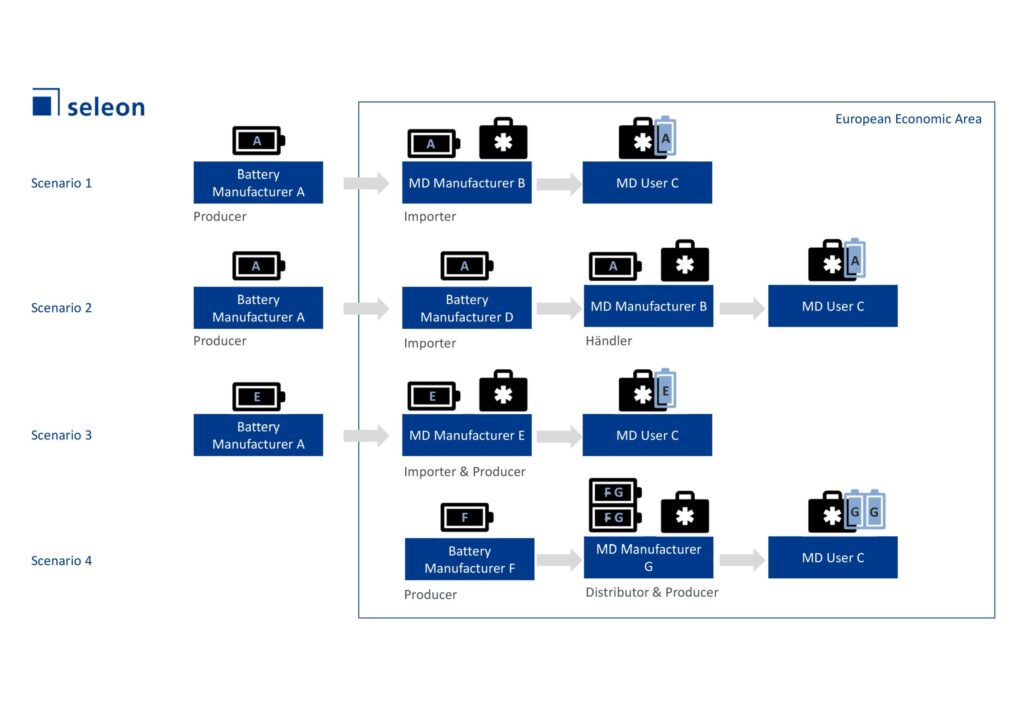
When is the medical device manufacturer considered to be the producer of a battery, and when is it considered to be an importer or distributor? Depending on the allocated role, different obligations apply to the medical device manufacturer. To illustrate this, seleon has defined four scenarios shown in the diagram below
Name Image: Roles of medical device manufacturers (MP manufacturers) in the Battery Regulation
What you should ask yourself: Are you the one placing the product on the market in the EU Economic Area (EEA) for the first time? In most cases, the manufacturer of the MP will buy a battery that is already on the market in the EEA and thus assumes the role of distributor (scenario 2). If he imports the battery from a producer in a third country, he becomes the importer (scenario 1). However, the MP manufacturer can also be assigned the role of producer under Article 44 if:
- he places a battery on the market or puts it into service under his own name or trademark (scenario 3),
- he modifies a battery already placed on the market or put into service in such a way that compliance with the relevant requirements of the Regulation could be affected (scenario 4), or
- changes the intended use of a battery already placed on the market or put into service.
Five battery categories – the portable battery in focus
Furthermore, a distinction is made between five battery types (portable batteries, industrial batteries, electric vehicle batteries, LV batteries, starter batteries). The category of portable batteries is particularly important for medical technology. It is irrelevant for the definition whether the batteries are permanently installed or replaceable, rechargeable or designed for disposable use. As long as the battery
- is encapsulated,
- weighs 5 kg or less and
- is not an industrial battery, an electric vehicle battery, an LMT battery or a SLI battery (starting, lighting and ignition batteries)
it is considered a portable battery. In turn, portable batteries are sometimes categorised as “general-purpose portable batteries” – i.e. batteries that are also used in everyday life and are specially designed for interoperability. In the following, we will only explain the obligations that apply to (general-purpose) portable batteries, as these are the main focus of medical technology.
What obligations do medical device manufacturers have with regard to portable batteries?
Are you a manufacturer of medical devices or in-vitro diagnostics and incorporate device batteries into your product according to the above definition? Then you have a number of obligations – an overview can be found in the table below. It is important to clarify in advance what your role is when installing portable batteries in your products (see chapter above). Depending on your role, you will have different obligations.
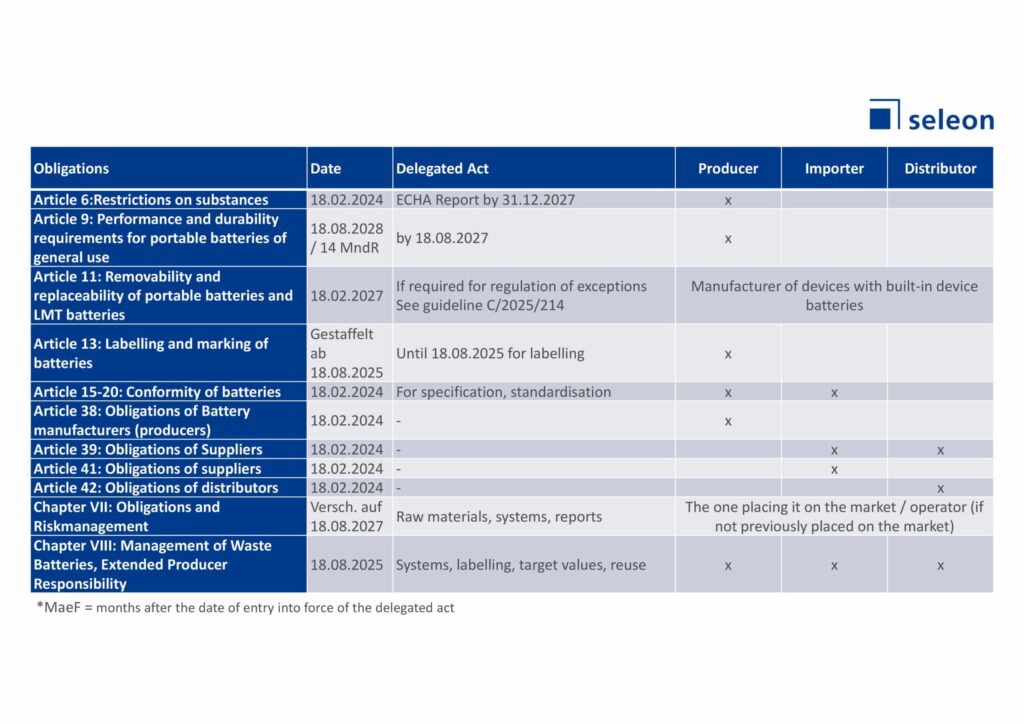
Caption: Obligations for device manufacturers with integrated batteries
As a producer, you are obliged to comply with the aforementioned Articles 6-20. If you are an importer or distributor, you must assure compliance of the producer with the obligations. This includes obligations regarding substance restriction, performance/shelf life, labelling and conformity assessment. All market participants in the EU must introduce market surveillance for their products and, if necessary, initiate corrective measures to ensure compliance with the obligations.
The omnibus for due diligence obligations along the supply chain provides a little more time to breathe: the start of application has been postponed by two years. This gives those placing the products on the markets and operators of batteries time to integrate strategies, control mechanisms and risk assessments for due diligence into their management systems and have them audited by notified bodies. The sticking point: in many places, there are still no notified bodies, as the authorities’ timeframe has also been postponed by one year to August 2026.
In addition, depending on the role, there are extended manufacturer responsibilities for collecting used batteries and financing the system. Even if you are not the one placing the battery on the market, but merely distribute them, you are obliged to take back waste batteries. However, authorised partners can assume obligations here.
Whether producer, importer or distributor: Article 11 “Removability and interchangeability of portable batteries” applies to all medical device manufacturers. So what do you need to consider when designing medical devices?
Obligation for removability of the battery by users also for medical devices?
From 18 February 2027, device batteries must be removable and replaceable by end users – without special tools, heat, solvents or manufacturer-specific tools. However, some exceptions may apply for manufacturers of medical devices and in-vitro diagnostics. This is because professional medical imaging and radiotherapy devices and in-vitro diagnostics are exempt from this obligation. Prerequisite: The device battery can be replaced by independent specialists.

Caption: Obligations for device manufacturers with integrated batteries
Even if you do not fall under this exemption, further exemptions to Art. 11 can be utilised if
- the devices are used in a humid environment,
- the continuity of the power supply is necessary and a permanent connection between the battery and the product is required to ensure user or device safety, or
- the main function of the product is to collect and deliver data and an interruption of the power supply jeopardises data integrity.
If an exception to the obligation is to be applied, this must be identified by means of a technical assessment and documented in the technical documentation. Guideline C/2025/214 (Annex I, Section 5) provides detailed provisions on this.
What do manufacturers need to do now?
Are you a manufacturer of a medical device with an integrated battery? Check the following: What is your regulatory role under the Battery Ordinance? Are batteries in your product removable? Depending on your role, the task list fills up quickly: perform conformity assessment, prepare technical documentation, report to registers, check labelling, prepare management systems for due diligence, implement extended producer responsibility…
Are you wondering what obligations await you as a manufacturer, importer or distributor?
Conformity assessment and technical documentation are causing you headaches?
Or are you faced with the task of adapting your medical device – be it for the interchangeability of the device battery or even an adaptation in the quality management system to fulfil new due diligence obligations?
seleon is your partner for these challenges.
With our experience, expertise and practical solutions, we provide you with reliable support on the path to greater safety and compliance.
Please note that all details and listings do not claim to be complete, are without guarantee and are for information purposes only.